Potassium Manganate Vii Formula
A 1 2 and 3 B 1 and 2 only C 1 and 3 only D 2 and 3 only. The equation for the reaction between manganateVII ions and sulfite ions is shown.
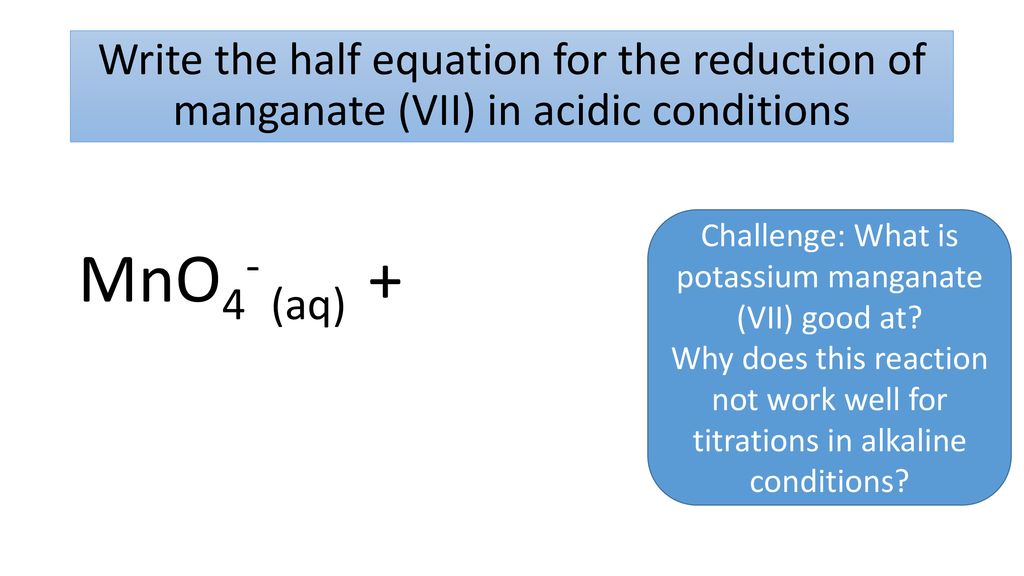
Challenge What Is Potassium Manganate Vii Good At Ppt Download
Investigating the kinetics of the reaction between potassium iodide and lead nitrate using microchemistry.
. 1 b Calcium is in Group II and chlorine is in Group VII of the Periodic Table. 3 Sulfur is oxidised from oxidation state 4 to 6. A student has a 10 g sample of each compound.
IAs involving molecular modelling. OH 5d hydroxy group because of broad peak at 3300 and one oxygen atom present in molecular formula. Calculate the molarity of the potassium manganateVII solution.
Potassium nonahydridorheniumVII potassium nonahydridorhenium2- f. Measure 30 cm 3 of acidified potassium manganateVII solution into one of the 100 cm 3 beakers and the same amount of water into the other. The uses of vegetable oils are extended using additives and chemical treatments.
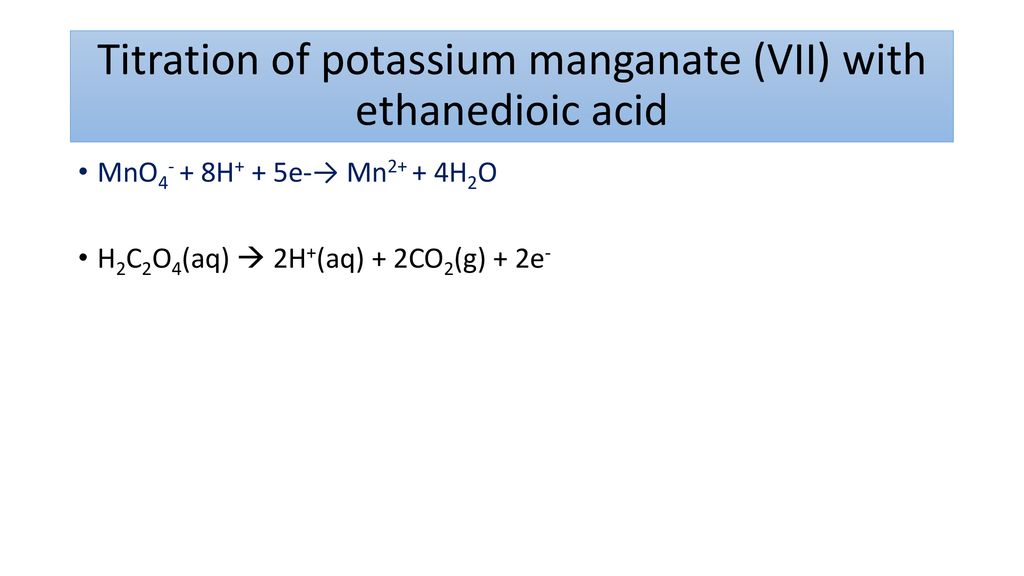
Potassium manganateVII Molecular Weight Molar Mass of Potassium permanganate. Using the reaction to test for carbon-carbon double bonds. 5112 use a back titration to determine the percentage of an active ingredient in an indigestion remedy link with Section 534.
Add one drop of the rhubarb filtrate to the potassium manganateVII solution and start the timer. NCERT Solutions for Class 12 Chemistry Chapter 8 The d and f Block Elements is a powerful study material that has answers to textbook exercises and important questions from the previous year and sample papers. Lithium sulfate VI Li 2 SO 4.
To investigate whether different iron complexes used in vitamin tablets are affected by pH using manganateVII titration. NCERT Solutions for Class 12 Chemistry Chapter 8 Free PDF Download. UMnO4 vH 2O wSO3 2 xMnO 2 ySO4 2 zOH Which statements are correct.
As you run the potassium manganateVII solution into the reaction the solution becomes colourless. CCEA Double award science. Potassium manganate VII KMnO 4.
As soon as you add as much as one drop too much the solution becomes pink - and you know you have reached the end point. The color of chemicals is a physical property of chemicals that in most cases comes from the excitation of electrons due to an absorption of energy performed by the chemical. Amount of copper in brass.
47 49 82 84 86 me a Give the formula of the ions responsible for each of the peaks. Hotwire hotels near debrecen. What is seen by the eye is not the color absorbed but the complementary color from the removal of the absorbed wavelengthsThis spectral perspective was first noted in atomic spectroscopy.
Stop the timer when the colour disappears and is the same as the plain water. Barium and oxygen chemical formulanational lampoons pledge this. Lanthanum chloride III LaCl 3.
970102 Cambridge International AS A Level Mark Scheme For examination SPECIMEN from 2022 UCLES 2019 Page 10 of 10 BLNAK PAGE. Enter the email address you signed up with and well email you a reset link. 532 titrate acidified potassium manganateVII with ironII and other reducing agents.
Potassium Permanganate - KMnO4 is the chemical formula of Potassium permanganate which is most commonly used as an oxidising agent in volumetric analysis. Give the formulae of the ions formed. 6 2020 06204220 3 The Periodic Table is a method of classifying elements.
Ayudando hoy para un mejor mañana. If you decide to dive right in to this section you might find it helpful to know that when applying nomenclature and formula rules most textbooks assume. The main disadvantage lies in the colour change.
1 u x z 2 Manganese is reduced to oxidation state 4. Explain in terms of number of outer shell electrons and electron transfer how calcium atoms and chlorine atoms form ions. These NCERT Solutions for Class 12 Chemistry are prepared based on the latest CBSE.
A Identify the element which is in Group VI and Period 4. Learn the properties structure and uses of Potassium permanganate KMnO4 Here. You see name and chemical formula next to temperature converted to the unit of your choice Celsius deegres kelvins Fehrenheits degrees etc.
11 The relative formula masses of four compounds are given. Whereas Ketones are more resistant to oxidation due to the lack of that particular hydrogen atom in them and only very strong oxidizing agents such as potassium manganate VII solution potassium permanganate solution are able to oxidize them. Hot AND concentrated.
A few drops of aqueous acidified potassium manganateVII solution are added to a sample of Y. Potassium manganateVII titrations are self-indicating. Carboxylic acids and esters are organic chemicals that occur naturally and can also be made from alcohols.
If an organic compound reacts with dilute alkaline potassium manganateVII solution in the cold to give a green solution followed by a dark brown precipitate then it may contain a carbon-carbon double bond. K 3 CrCl 6 or K 3. But equally it could be any one of a large number of other compounds all of which can be oxidised by manganateVII.
This becomes possible to do but in a harmful manner as the carbon-carbon bond is destroyed during. 2 mol of potassium manganate reacts with 5 mol of sodium ethanedioate SAMPEL 13 The mass spectrum for a sample of tetrachloromethane CCl4 is shown below. Solution Y is colourless.
511 Chemistry in medicine.

How To Write The Formula For Potassium Manganate Youtube
B8f78769 Potassium Manganate Vii 500g Philip Harris

Challenge What Is Potassium Manganate Vii Good At Ppt Download
Potassium Manganate K2mno4 Pubchem
0 Response to "Potassium Manganate Vii Formula"
Post a Comment